Gut-brain axis & neurostress: How the gut influences the brain – and how the lab shows the way to balance
The gut-brain axis is the connection between the central nervous system and the digestive system.
She works on neuronal, immunological, hormonal and microbial signaling pathways .
The flow of information is bidirectional – the gut communicates with the brain, and the brain with the gut.
Gut-brain axis & neurostress:
How the gut influences the brain – and how the laboratory shows the way to balance
From ORY Analysis
What does the gut-brain axis mean?
The gut-brain axis is one of the body's most fascinating communication systems.
It connects the central nervous system (brain and spinal cord) with the demential nervous system (the "gut brain") – via nerves, hormones, immune messengers and microbial signaling molecules.
This connection is bidirectional:
- The brain influences digestion, bowel movements, secretion, and barrier function.
- The gut sends signals back to the brain via immune substances, microbiota metabolites and the vagus nerve.
This creates a permanent exchange – a kind of biochemical dialogue between emotion and digestion.
This explains why stress affects the stomach, why anxiety triggers diarrhea, and why a healthy gut is often the basis of mental stability.
Why does the vagus nerve play a key role?
The vagus nerve is the most important communication pathway of this axis.
It is part of the parasympathetic nervous system, the "calming" nervous system, and functions as a biological data cable between the gut and the brain.
- Approximately 80% of its nerve fibers transmit information from the gut upwards – towards the brain.
- It conveys signals about stretching, saturation, inflammation, relaxation, and emotional reactions.
- When the vagus nerve is active, pulse, blood pressure and stress hormones decrease – the body regenerates.
Stress, on the other hand, inhibits vagal activity and activates the so-called HPA axis (hypothalamus-pituitary-adrenal axis), which releases cortisol, adrenaline and noradrenaline.
These stress hormones in turn alter intestinal motility, barrier function and the composition of the microbiome.
The result:
an “irritable bowel”, altered neurotransmitter production – and in the long term, disturbances in mood, sleep and energy.
gut-brain axis
This axis is the foundation of our emotional and physical balance.
Digestion, mood, sleep, appetite and even the immune system depend on it.
ORY Analysis takes a laboratory-based approach to the gut-brain axis, using modern, evidence-based analyses that make its function measurable.
The vagus nerve – the “social cable” between the gut and the brain
The vagus nerve is the central control organ of the gut-brain axis.
It provides relaxation, regeneration, and emotional stability.
- Approximately 80% of its fibers conduct information from the intestines upwards – not the other way around.
- It detects inflammatory mediators, microbiome signals, and hormones.
- An active vagus nerve lowers stress hormones, stabilizes pulse and blood pressure, and promotes digestion.
Vagal activity
Chronic stress inhibits vagal activity. This makes bowel movements sluggish, the intestinal barrier permeable, and the microbiome sensitive.
The result: a biological imbalance that becomes noticeable both physically and psychologically.
The microbiome – a hormone-active ecosystem
Our intestines are home to over 100 trillion microorganisms.
They influence:
- the formation of short-chain fatty acids (SCFAs) such as butyrate, acetate and propionate, which reduce inflammation and strengthen the intestinal barrier.
- the production of neuroactive substances – such as GABA, dopamine and serotonin precursors.
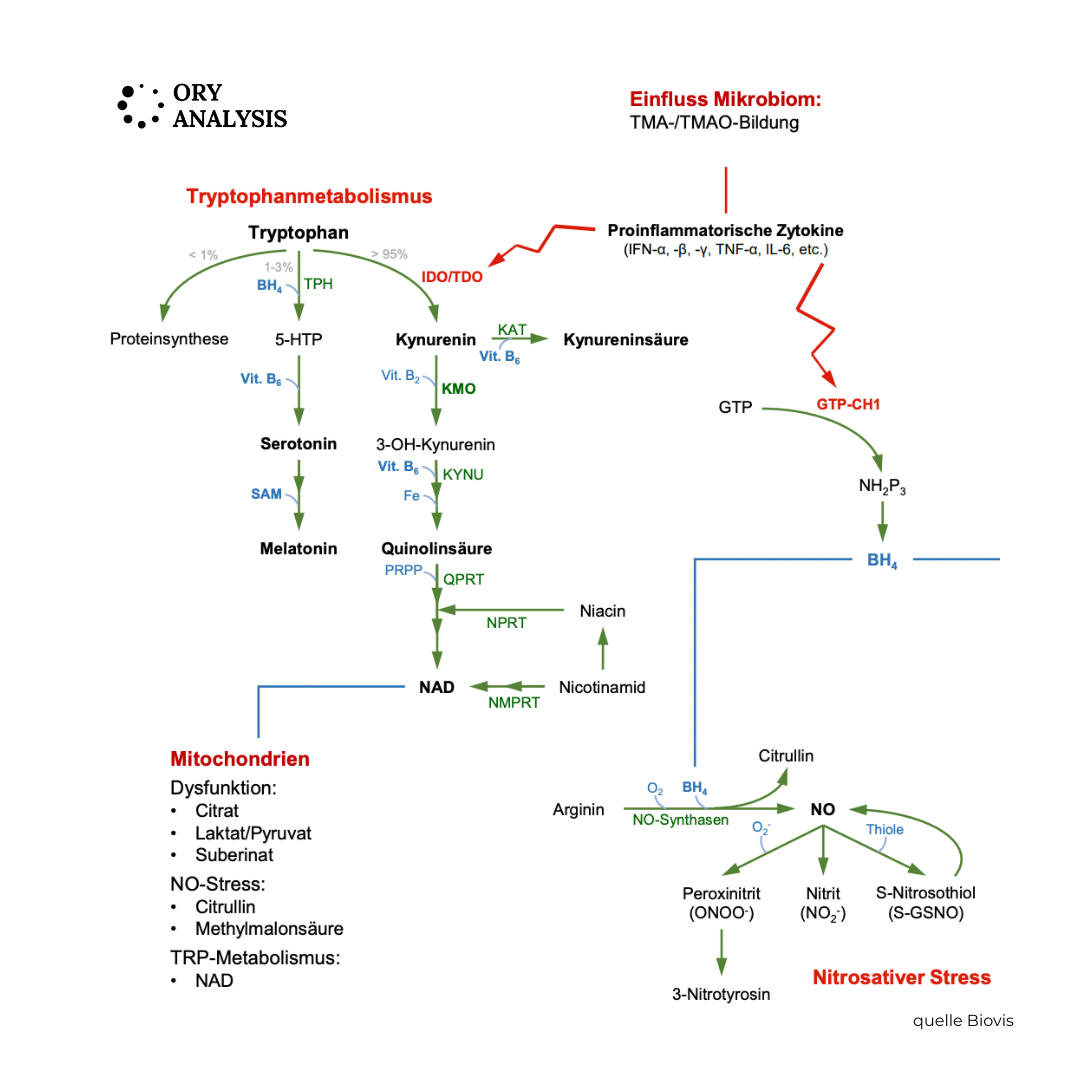
- the regulation of tryptophan metabolism, i.e. the balance between serotonin production and breakdown via the kynurenine pathway.
"cannot rest"
At ORY Berlin, this mechanism is taken into account in neurostress and microbiome diagnostics in order to understand why a person "cannot find peace" despite good nutrition and rest.
The tryptophan-serotonin metabolism
These processes explain why the microbiome influences not only our digestion, but also our mood, motivation, and cognitive performance.
Tryptophan-serotonin metabolism in the gut: Tryptophan is an essential amino acid – we must obtain it through our diet.
It is the precursor to serotonin, melatonin, and kynurenine. Approximately 95% of the body's own serotonin is produced in the gut – primarily in the enterochromaffin cells of the intestinal mucosa. Tryptophan metabolism can follow two different pathways:
The serotonergic pathway:
- Tryptophan → 5-Hydroxytryptophan (5-HTP) → Serotonin (5-HT)
This pathway promotes emotional stability, sleep, and intestinal motility.
It is promoted by sufficient tryptophan, healthy intestinal cells, B vitamins and a stable microbiome.
The kynurenergic path:
- Tryptophan → Kynurenine → Quinolinate / NAD⁺
This pathway is activated by the enzyme IDO (indolamine 2,3-dioxygenase) – especially during stress, inflammation and increased cortisol activity.
IDO activity
IDO “pulls” tryptophan away from the serotonin pathway – towards breakdown products, some of which have neurotoxic or immunomodulatory effects.
The role of IDO activity – the hinge between the immune system and the psyche
IDO activity is a key mechanism by which the immune system influences the brain.
It is primarily stimulated by inflammatory cytokines (e.g., interferon-γ, TNF-α, IL-6).
When IDO is active:
- The tryptophan level in the blood decreases,
- Serotonin synthesis in the gut and brain decreases,
- Instead, the proportion of kynurenine increases – a substance that forms neuroactive, partly pro-inflammatory metabolites.
This leads to a biochemical serotonin deficiency, even though the diet provides sufficient tryptophan.
This shift is called the "tryptophan-kynurenine shift".
Studies have shown it to be associated with depression, fatigue, irritable bowel syndrome, sleep disorders, and chronic stress.
How the laboratory makes these processes visible
A comprehensive picture of the gut-brain axis emerges from several laboratory parameters that complement each other.
1. Intestinal barrier and microbiome function:
- Zonulin (stool) – indicates permeability
- FABP2 (serum) – indicates mucosal damage
- SCFAs (chair) – demonstrate energy supply and anti-inflammatory activity
2. Neurotransmitters and metabolites (second morning urine):
- Serotonin → 5-Hydroxyindoleacetic acid (5-HIAA)
- Dopamine → DOPAC (3,4-Dihydroxyphenylacetic acid) • Noradrenaline/Adrenaline → Vanillylmandelic acid (VMS)
- The ratio of neurotransmitter to metabolite provides information about enzyme activity (MAO-A/B, COMT).
3. Tryptophan–kynurenine balance (optional in special diagnostics):
- Tryptophan, kynurenine and their ratio (kynurenine/tryptophan ratio) reflect IDO activity.
- An elevated quotient indicates an increased stress or inflammatory response that blocks serotonin production.
This creates a precise functional image:
How heavily stressed is the barrier?
How active is the neurotransmitter metabolism?
How does the tryptophan pathway react to stress and inflammation?
What clinical signs does increased IDO activity show?
An overactive IDO can explain many complaints that seem nonspecific at first glance:
- persistent tiredness or exhaustion,
- Mood swings, depressive mood, inner restlessness,
- Irritable bowel syndrome symptoms, bloating, loss of appetite,
- Sleep disturbances, reduced dream recall,
- Weakened immune system or frequent infections.
Therapeutic approaches in the ORY analysis concept
All these symptoms arise from a malfunction in tryptophan metabolism – stress and inflammation “consume” the tryptophan before it can be used to produce serotonin and melatonin.
ORY Analysis combines laboratory values with targeted, practical strategies:
- Diet: tryptophan-rich, low-inflammatory diet (oats, nuts, legumes, pumpkin seeds, fish, eggs).
- Strengthen the intestinal environment: Prebiotics and probiotics promote butyrate-forming bacteria and reduce inflammation.
- Stress reduction: breathing exercises, mindfulness, HRV biofeedback to activate the vagus nerve.
- Micronutrients: B6, B12, folate, magnesium, zinc – cofactors of serotonin and kynurenine synthesis.
- Exercise: moderate training lowers IDO activity and improves kynurenine metabolism.
- Sleep optimization: regular rhythms promote melatonin production.
- Repeat diagnostics: after 8–12 weeks to objectively assess progress.
Scientific context and limits
- IDO activation is a scientifically established mechanism of immune-neuro communication.
- It links chronic inflammation, stress response, and serotonin deficiency.
- The laboratory analyses provide functional, not psychiatric, diagnoses – they reveal biochemical patterns that are incorporated into therapy planning.
- ORY Analysis places great emphasis on methodologically correct, laboratory-specific interpretation – especially for zonulin and neurotransmitter tests.
Conclusion – Biochemistry as the language of health
The gut thinks along with it, stress contributes to the narrative – and the lab results tell the story.
The gut-brain axis and tryptophan metabolism are not abstract concepts, but measurable, modifiable systems.
ORY Analysis translates this complexity into clear, individualized diagnostics:
from the intestinal barrier via microbiome metabolism to neurotransmitter and IDO activity.
This reveals how stress, inflammation and diet shape mood – and how preventive medicine can restore balance.
Scientific studies
- Tsuji A, Ikeda Y, Yoshikawa S et al. “The Tryptophan and Kynurenine Pathway Involved in the Development of Immune-Related Diseases”. Int J Mol Sci. 2023;24(6):5742. Comprehensive review of tryptophan/kynurenine metabolism, focusing on the immune system, inflammation, and the neuro-/endocrine system. Relevance: Shows how IDO and kynurenine pathways are active in inflammatory diseases—important for interpreting laboratory values in the context of leaky gut or stress.
- Ilavská L, et al. “The kynurenine and serotonin pathway, neopterin…” Front Psychiatry. 2024; …↳ Investigates the effect of chronic stress on TRP (kynurenine) degradation and the serotonin pathway. Relevance: Directly relevant for neurostress diagnostics: explains how stress shifts serotonin production via IDO.
- Kim YK, et al. “Neuroinflammation and the Immune-Kynurenine Pathway”. 2020; …↳ Review of the mechanisms by which inflammation influences stress/anxiety responses via the kynurenine pathway. Relevance: Supports the link between the intestinal barrier/inflammation and neurotransmitter metabolites.
- Coplan JD, et al. “Early Life Stress and the Fate of Kynurenine Pathway …” PMC. 2021; …↳ Investigation of how early life stress affects the kynurenine pathway (with IDO activation) and thus long-term neurological effects. Relevance: Emphasizes the importance of lifestyle/early stress in prevention – important in the ORY-Berlin approach.
- O'Riordan K, et al. “Short chain fatty acids: the messengers from down below”. Front Neurosci. 2023; …↳ Review of gut SCFAs (short chain fatty acids) and their effects on the brain and nervous system. Relevance: To be used directly in the Microbiome/SCFAs section — shows the “gut → brain” mechanism.
- Review: “Microbiota–gut–brain axis and its therapeutic applications”. Nature. 2024; …↳ Modern review focusing on mechanisms of the gut-brain axis in neurodegenerative diseases. Relevance: Supports the general relevance of the topic in medicine and reinforces the prevention perspective.
- Review: “Review of microbiota gut brain axis and innate immunity”. Frontiers. 2023; …↳ Explores the interfaces between the microbiome, immune system, and brain – central role of barrier function and the vagus nerve. Relevance: Link between the barrier concept (“leaky gut”) and neurological imbalance.
- Review: “The Microbiota–Gut–Brain Axis: Key Mechanisms Driving …”. Life. 2025; 15(1):3.↳ Presentation of the latest mechanisms including the glymphatic system, microbiome, and microglial activation. Relevance: For future perspectives and explanation of more complex pathomechanisms.
- Review: “Gut-microbiome-brain axis: the crosstalk between the vagus nerve, gut …”. Nutr Rev. 2023; …↳ Focus on the vagus nerve, microbiome signaling, and metabolic regulation. Relevance: Strengthens the “vagus nerve” chapter in the article – demonstrates the anatomical/physiological connection.
- Muneer A. “Kynurenine Pathway of Tryptophan Metabolism in …”. Clin Psychopharmacol Neurosci. 2020;18(4):507-520.↳ Review article on the kynurenine pathway in psychiatric disorders (serotonin competition). Relevance: Supports the neurotransmitter metabolite theory – explains why elevated kynurenine levels are associated with depression, etc.

